
A pi bond between two atoms is formed only in addition to a sigma bond. Send comments, kudos and suggestions to us by email. The reason is that the overlapping of atomic orbitals can take place to a greater extent during the formation of a sigma bond, whereas overlapping of orbitals occurs to a smaller extent during the formation of a pi bond. This document and associated figures are copyright 1996-2022 by Rob Toreki or the contributing author (if any) noted above. This page was last updated Tuesday, March 31, 2015 Please visit our sponsor to thank them for supporting this site! Sigma bond metathesis plays a critical role in certain olefin polymerization reactions and is frequently encountered in reactions involving dihydrogen or silanes. The electrons that participate in a bond are referred to as electrons.
Due to the direct overlapping of the involved orbitals, sigma bonds are the strongest covalent bonds. This makes sigma bond metathesis a common reaction in lanthanide complexes as the lanthanides generally have only one common oxidation state (3+). A sigma bond is a covalent connection produced by collinear or coaxial overlapping of an atomic orbital in a line of the internuclear axis. Sigma bond metathesis is generally confined to those systems where oxidative addition is not a viable pathway. For example, oxidation addition in the example shown above would require the unlikely/impossible Sc 5+ intermediate,Cp* 2ScMeHR. When we say that the two hydrogen nuclei share their electrons to. Such complexes can not undergo an exchange of ligand by a pathway involving an oxidative addition followed by a reductive elimination. The simplest case to consider is the hydrogen molecule, H2. Sigma-bond metathesis is a common reaction with early transition metals that are in their highest oxidation state. Notice that this four-center mechanism does not involve a change in oxidation state. Sigma-bond metathesis is easier to explain with a drawing than with words:Īs shown in the drawing, a sigma-bonded ligand is replaced through reaction with the sigma bond of an incoming ligand.
#Sigma bonds plus
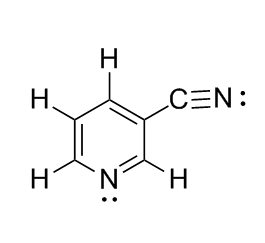
all single bonds between carbon and any other atom are sigma bonds (side note) double bonds between carbon atoms consist of a sigma bond plus a pi bond (side-side note) triple bonds between carbon atoms consist of a sigma bond plus 2 pi bonds. a pi bond is formed when two p orbitals overlap.
Visit our sponsor at Sigma Bond Metathesis a sigma bond is formed when two s orbitals overlap. Organometallic HyperTextBook: Sigma Bond Metathesis


 0 kommentar(er)
0 kommentar(er)
